I. Introduction
PSYCHOLEPTICS
PSYCHOANALEPTICS
PSYCHODYSLEPTICS
* * *
II. Therapeutic uses
PSYCHOLEPTICS
PSYCHOANALEPTICS
PSYCHODYSLEPTICS
III. The problem of control
STRUCTURE OF OICM *
THE TASKS OF OICM
OICM REQUIREMENTS
COMPARISON WITH OTHER COUNTRIES
EXPERTS' REPORTS
THE DRUG TRADE*
CONTROL OF PSYCHOTROPIC DRUGS
IV. General suggestions
Author: N. CAMPANINI
Pages: 13 to 34
Creation Date: 1967/01/01
" I fail to understand why man, a creature endowed with a mind and reason, resorts to artificial means in order to achieve a state of beatitude, when enthusiasm and will-power alone are sufficient to transport him into a supranatural existence."
BAUDELAIRE, <>
It is difficult to define a range of drugs which comprises the barbiturates, tranquillizers, amphetamines and hallucinogens, because they are not chemically related and they have different pharmacological properties.
However, in view of their common effect on the psychism, they may be described as psychotropic.
Abuse or prolonged use, of a considerable number of them can produce a state of dependence, corresponding to the new definition adopted by WHO. 1
Some degree of control of such drugs is therefore fully justified. Only the methods remain to be defined.
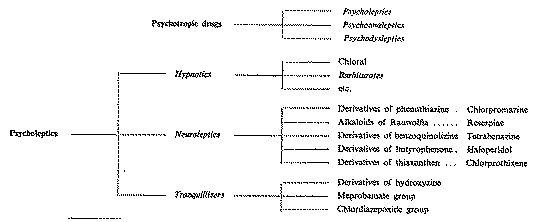
Before we proceed further with this study, the following general classification borrowed from Delay 2and Deniker 3 may help to clarify our ideas:
The original of this article is in French.
WHO Expert Committee on Addiction-Producing Drugs, Thirteenth Report, pp. 9-10 and Annex 1. Wld. Hlth. Org. techn. Rep. Ser., 1964, 273, 13-16. Eddy, N. B., Halbach, H., Isbell, H., and Seevers, M. H., " Drug dependence: its significance and characteristics ". Bull. Wld. Hlth. Org., 1965, 32, 721-733.
Delay, J., " Psychopharmacology and psychiatry - Towards a classification of psychotropic drugs by their nature and function ". Bulletin on Narcotics, Vol. XIX, No. l (1967). This work has been published in full in La Presse médicale, 74, 22, 30 April 1966.
Deniker, P., La Psychopharmacologie, Presses universitaires de France (1966)

|
For the sake of clarity, the four groups of drugs dealt with in this paper will be considered separately. Their potential dependence-producing effects will be indicated wherever possible.
|
Barbiturates
The result of a chance discovery by von Baeyer in 1863, the barbiturates were first introduced into clinical practice at the beginning of this century, first of all in the form of barbital, followed shortly afterwards by phenobarbital.
The barbiturates are a chemically homogeneous group of drugs with a cyclic ureide - barbituric acid - as their parent compound. The only differences relate to the side chains, which produce variations in both the duration and the time of onset of the effects.
Although the clinical use of barbiturates increased fairly rapidly, it was accompanied by certain drawbacks. For instance, it led to idiosyncracy phenomena, poisoning and, very soon, attempted suicides. Consequently the years 1920 to 1940 were marked, on both sides of the Atlantic, by a fierce battle in which advocates and opponents of the drugs confronted each other with equally valid arguments. Although the effectiveness of these substances in allaying anxiety and reducing nervous tension is beyond dispute, and although their hypnotic and anticonvulsant properties are still universally acclaimed, no one can deny their tendency to produce dependence.
In 1954, a leading article in the Lancet stated that although there was little proof of the toxic effects of the barbiturates, when administered in therapeutic doses, these drugs could nevertheless be regarded as capable of producing severe addiction.
In the same year, the British Medical Journal declared that the barbiturates met all the criteria of dependence-producing drugs, except that barbiturate addicts show a less marked tendency to increase the dosage, as compared with morphine addicts.
Repeated doses lead to chronic intoxication, manifested by a state of lethargy accompanied by tremor and dizziness. This may lead to a true ataxy, and even a derangement of the moral sense.
Ingestion of less than 0.4 g daily for more than six weeks will result in only minor withdrawal symptoms if the drug is abruptly discontinued. However, dosages of up to 0.6 g daily will already produce more pronounced symptoms, including anxiety, tremor and debility, while doses of 0.8 g or more daily will produce severe addiction. Withdrawal in the latter case will result in painful and serious phenomena such as convulsions (in about 75 % of cases) and psychosis phenomena accompanied by delirium (in about 60%).
Thus the repeated use of barbiturates leads to a certain degree of barbiturate-dependence, which is relatively difficult to cure. Disintoxication cures are invariably long, and require hospitalization, followed by a lengthy physical and occupational rehabilitation.
However, barbiturate-addiction is usually found in persons who show a predisposition to use drugs, whose psychological make-up is unstable or who suffer from Barbiturates, tranquillizers, amphetamines and hallucinogens, and their control in Switzerland 15 basic character disorders, e.g., psychopaths and hysterical persons.
This type of addiction is undoubtedly just as dangerous as that caused by true narcotics, mainly owing to the relative ease with which such drugs can be obtained, the extreme simplicity of their use and their low cost.
Barbiturates are also frequently used in conjunction with amphetamines, which help to counteract the lethargic and somnolent effect produced by the former. In turn, these drugs produce a state of agitation and insomnia which is counteracted by having fresh recourse to barbiturates. Thus gradually, a vicious circle sets in which cannot fail to have disastrous consequences.
Not uncommonly, the situation is aggravated by alcohol intake, which potentiates the effects of the barbiturates to such an extent that a small dose is liable in these circumstances to cause relatively serious intoxication.
Before concluding this introduction, we should like to mention a non-medical, but frequent and popular, use of these drugs, i.e., as a means of committing suicide. Acute poisoning is one of the most widespread causes of admission to hospital. However, whereas the mortality rate was still as high as 25 % at the end of the Second World War, today it is no more than some 2%, thanks to modern methods of treatment. This improvement in the prognosis is mainly due to plasmatic alkalization coupled with effective diuresis by means of mannitol and the derivatives of chloro-thiazide.
The reabsorption of the barbiturates through the renal tubules is greatly reduced by increased urinary pH (perfusion of trisaminol).
Furthermore, mannitol is an osmotic diuretic which, after being filtered by the glomerules, is not exposed to the action of the renal tubules. In this way, the gastric lavage and stimulation of the repiratory centres, which were formerly the basis of all treatment for acute intoxication, have become simple secondary therapeutic measures.
Neuroleptics
It is only about fifteen years ago that the group of drugs known as neuroleptics were first discovered and used for therapeutic purposes. They are nevertheless extremely important. In fact, their application in psychiatry has practically revolutionized hospital life, by enabling useful contacts to be made with the patient, even leading to his reintegration in everyday life.
Their main use in medicine is in the treatment of psychoses, of which schizophrenia is undoubtedly one of the most important. When administered in small doses, their effect is psycho-relaxing, but larger doses produce anti-psychotic effects. Unlike hypnotics, in particular the barbiturates, they do not produce loss of consciousness. Hence it is safe to administer comparatively large doses, particularly if the aim is to change the patient's whole personality.
Again, unlike the barbiturates, they do not appear to produce dependence. Nevertheless, they potentiate the effects of the latter and alcohol, as well as of the analgesics. For this reason, a certain amount of caution is essential when they are used in the out-patient department.
The increase in psychic and psychosomatic disorders as a direct result of our modern way of life had led to a considerably higher consumption of neuroleptics. In fact, these medicaments are often taken without any valid medical justification, in a way impossible to control and without any authoritative advice. They can therefore cause serious intoxication and even suicides. Their relative lack of toxicity, fairly wide therapeutic properties and habitually low posology may lead to mistakes being made. Although neuroleptic tablets usually contain only 10 to 25 mg of active substance - unlike the barbiturates, where the unit dose varies between 100 and 200 mg - the prescriptions usually call for 2 to 3 tablets, to be taken several times a day. The patient thus has access to a relatively large number of tablets; it is not unusual for him to have a supply of 100 or 200 in hand. Such a quantity, which is easily lethal, may account for a number of suicides, especially as their massive administration is made still easier by the fact that they are sugar-coated.
Chemically speaking, neuroleptics do not have quite the same unity of origin as the barbiturates. They include, for example, the important group of the pheno-thiazines, of which chlorpromazine is one of the chief representatives, and the one most used, the derivatives of thiaxanthen, mainly chlorprothixene, the chemical structure of which shows a certain analogy with that of the preceding group, and finally the alkaloids of Rauwolfia, headed by reserpine, the importance of which needs no further demonstration.
These few examples are enough to show the lack of chemical homogeneity of all these derivatives. Although their general action is identical, their pharmacological properties are not the same for each group, any more than they are for the individual substances within each group. Accordingly, not only do the phenothiazines not act in the same way as the alkaloids of Rauwolfia, for instance but, each of them has a different specific action in relation to specific types of disorder.
Tranquillizers
The term "tranquillizers" is used here in an extremely wide sense to cover a vast complex of widely varying drugs such as neuroleptics, tranquillizers proper and antidepressants. Since each group has entirely different properties, it is necessary to consider them separately.
This section will be confined to tranquillizers proper, disregarding the terminology adopted at the beginning of our introduction, according to which all drugs whose sedative action is not hypnogenic are grouped under this heading.
Like neuroleptics, tranquillizers are a recent discovery. Their chemical structures bear no relation to each other. Among tranquillizers, the meprobamate group, first of all, and then more recently the chlordiazepoxide group are of considerable therapeutic interest. They are mainly characterized by a sedative and anxyolitic action, free of hypnotic effects, at least when administered in normal doses. On the other hand, it is not excluded that some of them, when used with a higher posology, may reveal similar properties. However, they differ from the conventional sedatives such as the barbiturates, by the wide gap which separates the sedative dose from the hypnotic dose. Unlike the neuroleptics, they do not have any antipsychotic action. They are also liable to cause muscular relaxation, apart from a general sedation. They do, however, show certain analogies with the barbiturates: like the latter, they may in certain cases produce addiction depending, obviously, on the dose and the frequency with which they are taken. In view of the large amounts to which a patient may have access, however, the danger of habituation is very real, and the danger of addiction no less so.
Stimulants of mood
These have the same characteristics as the two previous groups: no chemical relationship and pharmacological properties varying with each category of derivatives.
As in the case of tranquillizers and neuroleptics, the discovery of psychoanaleptics dates back a few years only and they are greatly valued in medical practice. Their main characteristic is to stimulate the mood and correct states of depression. Unlike the amphetamines, they do not excite: they modify the mood, but do not mask temporarily a depressive condition which is characterized by sadness, pessimism, moral pain accompanied by self-denigration or self-accusation, reduced energy and will-power.
Psychoanaleptics may be divided into two groups, the derivatives of imipramine and the inhibitors of monoamine oxidase. Of the latter, the hydrazine group is the one most commonly used.
As often happens with so many drugs, the antidepressant action of the second group was discovered quite by chance. They were first used therapeutically for tuberculostatic purposes, and their stimulating action only emerged as a side effect in connexion with the treatment of tuberculosis. Like the other tranquillizers, using the term in the broad sense, they potentiate the effect of certain drugs such as the amphetamines, the barbiturates and alcohol. Consequently, they may in these circumstances be a source of danger which must be guarded against. Furthermore, since they are used over a fairly long period, it is possible that they may also produce dependence.
Amphetamines
The amphetamines, or sympathomimitic amines, are a chemically relatively homogeneous group and are pharmacologically related to adrenaline and ephedrine.
With few exceptions, the drugs in this group are derived from phenyl-aminopropane or amphetamine, which, as is well known, excite the central nervous system. They therefore produce a stimulation of mood. But this stimulation is achieved in quite a different way from that caused by MAO inhibitors, where action is slow and the effect obtained without any psychosomatic excitation. The sensation of fatigue disappears and a euphoric action is manifested.
Such drugs obviously produce serious dependence. Their abuse - often found in combination with the abuse of barbiturates - causes a vicious circle which it is difficult to break. The danger is twofold: firstly, the resulting excitation may lead to alarming psychic phenomena, and secondly, the feeling of depression which sets in with the cessation of medication is often very intense and of long duration. Inevitably, it leads to a condition in which the victim craves more drugs. Amphetamine addiction may therefore be compared with barbiturate addiction. In one respect, it is even more dangerous, in view of the very powerful effect of amphetamines on the central nervous system, even when administered in relatively small doses.
Hallucinogens
These drugs deserve special mention in view of their very limited therapeutic use and their violent effect on the psychism. For this reason, their use is virtually Barbiturates, tranquillizers, amphetamines and hallucinogens, and their control in Switzerland 17 confined to psychiatric investigation. They are of different origins: mescaline is obtained from the South-American cactus peyotl; bufotenine from piptadenia; psilocybin and psilocin from the Mexican mushroom Psilocybe mexicana; while ergot provides lysergic acid, from which its diethylamide is prepared, the latter being better known as lysergide or LSD.
While their chemical unity is not absolute, they nevertheless have one common characteristic, namely an indolic core.
Since LSD is by far the most important of these substances, we propose to deal here with it alone. Of Swiss origin, it was discovered quite by chance by Hofmann, who synthetized it for the first time in 1938 in the laboratories of Sandoz S.A., Basle, while working on ergotic alkaloids. For five years the discovery did not give rise to any special research, and it was not until 1943 that Hofmann himself discovered its pharmacological properties by experiencing, when engaged on some chemical experiments, physical and psychic disorders of the type described below:
I. Physical disturbances
Motor affections: lassitude, sensory disorders, ataxic gait, impairment of speech, deterioration of writing, etc.
Vegetative symptoms: indisposition, nausea, a feeling of emptiness, buzzing in the ears, headache, perspiration, accelerated heartbeat; in some cases, slowing of respiration and lowering of blood pressure.
Disorders of visual preception: the appearance in a dark room of polychrome visions and pseudo-hallucinations in broad daylight.
Auditory hallucinations: magnification of sound to the point where a mere murmur may become as unbearable as the noise of a cataract.
Disorders of smell and taste.
Disorders of the sense of touch and deep sensibility.
II. Psychic disturbances
These are even more serious than the physical disturbances listed above. They include:
Disturbances of the perception of space and time, loss of sense of ego and personality disturbances.
Disturbances in logical thinking, reduced powers of concentration.
Changes in mood varying from euphoria to depression.
Modification of memory function.
Clearly, the use of drugs which are liable to produce such disorders and modify the psychism to such an extent cannot be entrusted to non-experts. To meet this danger, therefore, a decision was recently taken in Switzerland, as will be seen further on in the chapter dealing more specifically with control.
It would certainly be most interesting to know the world production of the drugs discussed in this article, to work out the percentage used for medical purposes and carry out comparative investigations at the Swiss level.
Unfortunately, such statistics, covering particular groups of substances, exist only in an extremely fragmentary fashion at the world level, and so far not at all in Switzerland. As for statistics of global production, these are of only relative interest within the context of this survey.
However, the problem has not been ignored by the Swiss authorities, for in 1965 they appointed Professor Kielholz to make a general study of the production and consumption of these drugs in Switzerland, with the object of finding whether or not there was any abuse of these drugs in our country, and thus shedding a more objective light on the subject.
The results of this study will not be known until the second half of 1967 and we will therefore make no attempt to hazard an estimate here - which at this stage would be mere speculation anyhow, most certainly incomplete and even false. However, without giving any specific figures, we can nevertheless affirm in its defence that the amount of licit LSD production by Swiss industry is too small to constitute anything approaching a world danger.
Comparative table of the chemical derivatives and pharmaceutical preparations derived therefrom, as used in Switzerland and throughout the world *
|
Barbiturates |
Tranquillizers |
Amphetamines |
|
|---|---|---|---|
|
1. Number of chemical derivatives used throughout the world
|
55 | 110 | 9 |
|
2. Number of chemical derivatives used in Switzerland
|
19 | 45 | 5 |
|
3. Kinds of pharmaceutical preparations marketed through out the world
|
921 | 1,669 | 397 |
|
4. Kinds of pharmaceutical preparations marketed in Switzerland
|
53 | 76 | 24 |
|
5. Ratio, expressed as a percentage between items 2 and 1
|
34.5 | 40.9 | 55.6 |
|
6. Ratio, expressed as a percentage between items 4 and 3
|
6 | 4.6 | 6 |
In the first part of this study we have adopted Delay and Deniker's "standard" classification. For practical reasons, and also because of the usage in Switzerland, the classification in the following table is slightly different - any classification in this field will in any event always be provisional, the basic aim being to ensure clarity. In the following table, therefore, we have also included the neuroleptics and mood stimulants under tranquillizers.
The information presented below consists of: (1) A table comparing the use in Switzerland and throughout the world of chemical substances and pharmaceutical preparations derived therefrom, the ratio being expressed as a percentage; (2) A table containing a list of the various preparations marketed in Switzerland for each group of substance, under their chemical, international (or other) and trade names.
|
Chemical formula |
Common international or other name |
Trade name |
|---|---|---|
|
(+) -2-amino-1- phenylpropane
|
Amphetamine Amphaetaminum Amphaetaminum sulfuricum Rac-desoxynorephedrine
|
Adiparthrol Aktedron Amphaetamine Benzedrine Biphetamine Corydrane Ortedrine Pento-adiparthrol Stenamine Sympamine
|
|
(+) -2-amine- 1- phenylpropane
|
Dexamphetamine Dextroamphetamine sulfate Dexamphetamine sulfate Dexamphaetaminae sulphas
|
Biphetamine Coffadyn Desoxyn Dexamphaetaminum Dexamyl Dexedrine Maxiton Obolip Pentoadiparthrol Scambelline Stenamine Sympamine
|
|
(+) -1-phenyl-2- methylaminopropane
|
Methamphetamine Methamphetamine hydrochloride Methylamphetamine
|
Adiparthrol Desoxyn Pervitine Stenamine Tonedron
|
|
phenyl-2-piperidineacetic acid methyl ester
|
Methylphenidate Methylphenidate hydrochloride
|
Plismaine Ritaline Serpatonil
|
|
3-methyl-2-phenylmorpholine
|
Phenmetrazine Phenmetralinum Oxazimedrine Phenmetrazinum hydrochloride
|
Cafilon Preludine
|
|
Chemical formula |
Common international or other name |
Trade name |
|---|---|---|
|
5-allyl-5-(2-bromoallyl)-barbituric acid
|
Brallobarbital
|
Vesparax
|
|
5-allyl-5-(2-cyclopenten-l-yl)barbituric acid
|
Cyclopentobarbital
|
Barecal Cyclopal
|
|
5-allyl-5-sec-butylbarbituricacid
|
Butalbital Allybarbituric acid Alisobumalum Talbutal Tetralobarbital
|
Cafergot Sandoptal
|
|
5-allyl-5-isopropylbarbituricacid
|
Aprobarbital Allypropymalum Allopropylbarbital
|
Plexonal Somnifene
|
|
5-allyl-5-(1-methylbutyl)barbitiruc acid
|
Secobarbital Secobarbital sodium Meballymalum Quinalbarbitone sodium Soluble secobarbital
|
Immenoctal Secorbarbital Seconal Tuinal Vesparax
|
|
5-(2-bromoallyl-5-isopropyl) barbituric acid
|
PropallylonalI bomalum
|
Quietal
|
|
5-butyl-5-ethylbarbituric
|
Butobarbital
|
Butisol
|
|
acid
|
Tridomal
|
|
|
5-(1-cyclohexen-1-yl)-5-ethylbarbituric acid
|
Cyclobarbita lCyclobarbitalum calcium Cyclobarbiton eHexemalcalcium
|
Cyclobartital Phanodorme Phanodorme calcium Proponal Quadronox Sanox
|
|
5-(1-cyclohexen-l-yl)-l,5-dimethylbarbituric acid
|
Hexobarbital Hexobarbitalum Enhexymalum
|
EvipanTobinal
|
|
5,5-diallylbarbituric acid
|
Allobarbital Allobarbitalum Diallymalum
|
Asmac Cibalgine Dial Somnocodal
|
|
5,5-diethylbarbituric acid
|
Barbital Barbitalum Barbitone Diemal
|
Barbital Calmine Neurinase Peralga Plexonal Veramon Veronal
|
|
Chemical formula |
Common international or other name |
Trade name |
|---|---|---|
|
5,5-diproplybarbituric acid
|
Propylbarbital
|
Proponal
|
|
5-ethyl-5-cycloheptenyl-barbituric acid
|
Heptabarbital Heptabarbitone
|
Medomine
|
|
5-ethyl-5-isopentylbarbituricacid
|
Amobarbital Amylobarbitone Pentymalum
|
Amytal Dexamyl Eunoctal Tuinal
|
|
5-ethyl-5-(l-methylbutyl)barbituric acid
|
Pentobarbital Pentobarbitalum solubile Pentobarbiton Mebumal natrium
|
Barecal Carbrital Nembutal
|
|
5-ethyl-5-(1-methylbutyl)-2-thiobarbituric acid
|
Thiopental Thiopentalum natrium Thiopental sodium Thiomebumalum Penthiobarbital
|
Pentothal
|
|
5-ethyl-N-methyl-5-phenylbarbituric acid
|
Methylphenobarbital Methylphenobarbitalum Enphenemalum Mephobarbital
|
Prominal
|
|
5-ethyl-5-phenylbarbituricacid
|
Phenobarbital Phenobarbitalum Phenemalum
|
Alepsal Anirrit Barecal Corosedine Deriminal Dilatrane Dolviran Epanal Eupaco Gardenal Hydantal Limunal Luminalettes Myocardon Natisedine Perphyllon Phenobarbital Plexonal Priscophene Quietal Tensedine
|
|
5-phenyl-5-allyl-barbituric acid
|
Alphenal Phenallymal Phenallymalum
|
Efrodal
|
|
Chemical formula |
Common international or other name |
Trade name |
|---|---|---|
|
2-acetyl-l-(3-dimethylaminopropyl)
|
Acepromazine
|
Plegicyl
|
|
phenothiazine
|
Acetylpromazine
|
|
|
10-[2,3-bis(dimethylamino) propyl]
|
Aminopromazine
|
Quietal
|
|
phenothiazine
|
Proquamezine
|
|
|
Tetrameprazine
|
||
|
3-phenyl-propylcarbamate
|
Phenprobamate
|
Gamaquil
|
|
Phenprobamatum
|
||
|
Phenbromatum
|
||
|
Proformiphen
|
||
|
2-carbamoyloxymethyl-2-
|
Carisiprodol
|
Somalgit
|
|
isopropylcarbomoyl-oxymethyl
|
Carisoprodatum
|
|
|
pentane
|
Isomeprobamate
|
|
|
5-chloro-2-benzoxazolidone
|
Chlorzoxazone
|
Paraflex
|
|
Chloroxazone
|
||
|
7-chloro-l,3-dihydro-l-methyl-
|
Diazepam
|
Valium
|
|
5-phenyl-2H-1,4 benzodiazepin-2-one
|
||
|
2-chloro- 10-(3-dimethylaminopropyl)
|
Chlorpromazine
|
|
|
phenothiazine
|
Chlorpromazine hydrochloride
|
Largactil
|
|
1-(4-chlorodiphenylmethyl)-4-(3-
|
Meclozine
|
Itinerol
|
|
methylbenzyl)-piperazine
|
Histametazine
|
Peremesine
|
|
Meclizine hydrochloride
|
Postafene
|
|
|
1-p-tert-butylbenzyl-4-(p-chloro-
|
Buclizine
|
Longifene
|
|
phenylbenzyl)piperazine
|
Buclizinum
|
Postafene
|
|
Histabutizine
|
||
|
2-chloro-10-[3-(4-methyl-1-
|
Prochlorperazine
|
Stemetil
|
|
piperazinyl) propyl]phenothiazine
|
Chlormeprazine
|
|
|
Prochlorpemazine
|
||
|
2-cyano- 10- [3-(4-hydroxypiperidino)
|
Periciazine
|
Neuleptil
|
|
propyl]phenothiazine
|
Propericiazine
|
|
|
10,11-dihydro-N,N-dimethyl-5H-
|
Amitriptyline
|
Laroxyl
|
|
dibenzo(a,d) heptaleneΔ5,γ-
|
Amitryptylinum
|
Tryptizol
|
|
propylamine
|
||
|
2-methyl-2-propyl-l,3-
|
Meprobamate
|
Eubamate
|
|
propanediol dicarbamate
|
Procalmidol
|
Meprobamatum
|
|
Meprocalm
|
||
|
Meprodil
|
||
|
Meprolettes
|
||
|
Miltown
|
||
|
Oasil
|
||
|
Pertranquil
|
||
|
Probamyl
|
||
|
Proponal
|
||
|
Quaname
|
|
Chemical formula |
Common international or other name |
Trade name |
|---|---|---|
|
10-(2-diethylaminoethyl)
|
Diethazine
|
Diparcol
|
|
phenothiazine
|
||
|
2-diethylaminoethyl 1-
|
Caramiphen
|
Parpanit
|
|
phenylcyclopentane-carboxylate
|
Caramiphen hydrocloride
|
Taoryl
|
|
3,3-diethyl-2,4-dioxotetrahydro-
|
Pyrithildione
|
Persedon
|
|
pyridine
|
Pyrthyldion
|
|
|
3,3-diethyl-5-methyl-2,4-
|
Methyprylone
|
Nodular
|
|
piperidedione
|
Methyprylon
|
|
|
p-butylthiodiphenylmethyl-2-
|
Captodiane
|
Covatine
|
|
dimethylaminoethyl-sulfide
|
Captodiane hydrochloride
|
|
|
10,11-dihydro-5-(3;dimethylamino-2-
|
Trimipramine
|
Surmontil
|
|
methylpropyl)-5H-dibenz [b,f] azepine
|
Trimiprimine
|
|
|
5-(3-dimethylaminopropyl)-10,11-
|
Imipramine
|
Nisorex
|
|
dihydro-5H-dibenz [b.f]-azepine
|
Imipraminum
|
Tofranil
|
|
10-(2-dimethylaminipropyl)
|
Promethazine
|
Avomine
|
|
phenothiazine
|
Promethazine hydrochloride
|
Fenerodine
|
|
Proazamin chloride
|
Phenergan
|
|
|
10-(3-dimethylaminopropyl)
|
Promazine
|
Prazine
|
|
phenothiazine
|
Promazin hydrochloride
|
Tyzine
|
|
2-(4-chlorophenyl)-3-methyl-
|
Chlormezanone
|
Trancopal
|
|
4-metathiazanone-l,l-dioxide
|
Chlormezanonum
|
|
|
Chlormethazanone
|
||
|
2-diethylaminoethyl
|
Adiphenine
|
Transentine
|
|
diphenylacetate
|
Adipheninum
|
|
|
2-diphenylmethoxy-N,N-
|
Diphenhydramine
|
Allerga
|
|
dimethylethylamine
|
Diphenhydramin hydrochloride
|
Benadon
|
|
Diphenylhydramine
|
Benadryl
|
|
|
Benylin
|
||
|
Caladryl
|
||
|
Nautamine
|
||
|
3,4,5 trimethoxybenzoic acid
|
Reserpine
|
Adelphan
|
|
ester of methyl reserpate
|
Reserpin
|
Barecal
|
|
Eubamat
|
||
|
Modenol
|
||
|
Ondasil
|
||
|
Pacyl-R
|
||
|
Sedaraupin
|
||
|
Serpasil
|
||
|
Serpatonil
|
|
Chemical formula |
Common international or other name |
Trade name |
|---|---|---|
|
3,4,5-trimethoxycinnamic acid
|
Rescinnamine
|
Anaprel
|
|
ester of methyl reserpate
|
Reserpyle
|
Modenol
|
|
Raupyrol
|
||
|
Triraupine
|
||
|
3-ethyl-3-phenyl-2,6-dioxopipe-
|
Gluthethimide
|
Doridene
|
|
ridine
|
Ondasil
|
|
|
2-(ethylthio)-10-[3-(4-methyl-1-
|
Thiethylperazine
|
Torecan
|
|
piperazinyl) propyl] phenothiazine
|
Thiethylperazinum
|
|
|
2-oxo-3-isobutyl-9,10-dimethoxy-
|
Tetrabenazine
|
Nitoman
|
|
1,2,3,4,6, 7-hexahydro-11bH-
|
||
|
benzo ? quinolizine
|
||
|
(-)- 10-(3-dimethylamino-2-methyl-
|
Levomepromazine
|
Minozinan
|
|
propyl)-2-methoxy-phenothiazine
|
Methotrimeprazine
|
Nozinan
|
|
Methoxyphenothiazine
|
||
|
10-(3-dimethylaminopropyl)-2-
|
Methopromazine
|
Quietal
|
|
methoxyphenothiazine
|
Methoxypromazine
|
|
|
3-(2-methoxyphenoxy)-1,2-
|
Methoxypropanediol
|
Resyl
|
|
propanediol
|
Tolserone
|
|
|
10-(3-dimethylaminopropyl-
|
Alimemazine
|
Theralene
|
|
2-methyl) phenothiazine
|
Alimemazinum
|
|
|
Trimeprazine
|
||
|
Methylpromazine
|
||
|
3-methyl- 1-pentyn-3-ol
|
Methylpentynol
|
Dormison
|
|
Methylpentynolum
|
Oblivon
|
|
|
Quietal
|
||
|
3-(o-methylphenoxy)-l,2-
|
Mephenesine
|
Decontractyl
|
|
propanediol
|
Mephenesin
|
Tolserol
|
|
Glykresinum
|
||
|
Cresoxydiol
|
||
|
Cresoxypropandiol
|
||
|
Toloxypropandiol
|
||
|
10-[2-( 1 -methyl-2-piperidyl)
|
Thioridazine
|
Mellerettes
|
|
ethyl]-2-(methylthio)
|
Thioridazinum
|
Melleril
|
|
phenothiazine
|
||
|
2-methyl-3-o-tolyl-4 (3H)
|
Methaqualone
|
Methasedil
|
|
quinazolinone
|
Tuazolon
|
Toquilone
|
|
N-p-methoxybenzyl-N', N'-
|
Thonzylamine
|
Anahist
|
|
dimethyl-N-2-pyrimidinethylene
|
Thonzylamine hydrochloride
|
Novohetramine
|
|
diamine
|
||
|
2-(o-benzylphenoxy) ethyl-
|
Phenyltoloxamine
|
Super Anahist
|
|
dimethylamine
|
|
Chemical formula |
Common international or other name |
Trade name |
|---|---|---|
|
5- [(o-methoxyphenoxy) methyl]
|
Mephenolaxone
|
Control-Om
|
|
-2-oxazolidinone
|
Dorsiflex
|
|
|
Dorsilon
|
||
|
7-chloro-2-methylamine-5-
|
Chlordiazepoxide
|
Librax
|
|
phenyl-3H- 1,4-benzodiazepine-
|
Clopoxide
|
Librium
|
|
4-oxide
|
Methaminodiazepoxide
|
|
|
1-(p-chlorobenzhydryl)-4
|
Hydroxyzine
|
Atarax
|
|
[2-(2-hydroxyethoxy)ethyl]
|
Hydroxyzine hydrochloride
|
Vesparax
|
|
piperazine
|
||
|
(±) 1-(p-chlorodiphenylmethyl)
|
Chlorcyclizine
|
Di-Paralene
|
|
-4-methylpiperazine
|
Chlorcyclizine hydrochloride
|
Trihistan
|
|
Histachlorazine
|
||
|
Trans-2-chloro- 10-(3-dimethyl-
|
Chlorprothixene
|
Taractan
|
|
aminopropyliden) thiaxanthen
|
In view of the extreme variety of these substances, their therapeutic uses will be studied separately for each class of drugs and explained as far as possible by reference to a few simple notions of physiology.
Barbiturates
Owing to their effect on the central nervous system, the barbiturates are used extensively throughout the world for medication purposes. However, their consumption has tended to become stabilized and has even declined in Switzerland since the introduction of tranquillizers into therapeutic practice.
As already indicated, these drugs were first used in medical practice at the beginning of this century, about the year 1903, when diethylbarbituric acid or barbital was synthesized. Their initial application was in surgery, but it was accompanied by certain drawbacks because of slow elimination. This factor was decisive in the search for less toxic derivatives which would decompose more rapidly in the organism, and led to the discovery, in succession, of allobarbital, heptobarbital and hexobarbital, to mention but a few.
Briefly, the barbiturates may be divided into three groups, based on the duration of their action:
long-acting: barbital, phenobarbital;
medium-acting: amobarbital, dial, cyclobarbital, heptobarbital;
short-acting: hexobarbital, secobarbital, butobarbital, pentobarbital.
Taken in combination with other drugs, such as the analgesics, the barbiturates posesss useful sedative properties, due to their synergic action.
Biologically, these depressents of the central nervous system reduce the functional activity of the living cells, according to a very regular progressive pattern.
Their first effect is to paralyse the brain, which determines both insensibility to pain and loss of consciousness. However, at this stage of analgesia, the muscular reflexes continue to function. At this level, the barbiturates are used medically in minor surgery and obstetrics. Their depressent effect at this stage is confined to the brain, while the spinal cord continues to function normally.
In the next stage, known as anaesthesia, the action of the drugs gradually extends to the spinal cord, resulting in muscular relaxation and the disappearance of reflexes - voluntary or other - without, however, affecting the basic mechanisms of the organism, such as respiration and circulation.
Finally, if the drug is left to act, its paralysing effect on the nerve centres extends to the medulla oblongata, producing a state of collapse, followed by a slowing down of the respiratory rhythm, anoxia, cyanosis, a lowering of the blood pressure and finally, death.
Accordingly, the barbiturates are used in medical practice for various purposes, which may range from sedation to hypnosis, depending on the dose. These therapeutic purposes may be summarized as follows:
to produce hypnosis;
to allay anxiety;
to reduce nervousness;
to reduce emotional tension;
as anti-convulsants (treatment of epilepsy);
to produce anaesthesia and pre-anaesthesia;
as instruments of research and diagnosis: they enable organic disorders to be distinguished from functional disorders, and functional disorders from similated disorders.
Another interesting but lesser known use of sedatives such as the barbiturates may appear to some extent paradoxical. This is their use to overcome states of chronic fatigue such as are common among intellectuals, and which are connected with peripheric circulatory disorders. Whereas all medication of an excitatory nature remains ineffective, small doses of the barbiturates may reduce circulatory hyperexcitability.
Neuroleptics
The neuroleptics have the effect of modifying the psychism. Although their mode of action differs for each neuroleptic agent, it is characterized by its relation with the metabolism of the brain monoamines, and with two neurophysiological units, the reticular formation and the limbic system.
Although used for the treatment of identical syndromes, the neuroleptics do not, like the barbiturates, produce a narcotic state with loss of consciousness. Recent electro-physiological and biochemical discoveries provide us with a better understanding of the mechanism of their action. Unlike the hypnogenics, they do not directly affect the reticular formation, as is shown by the implantation of electrodes, and they block the sensory afferents.
These two reasons explain, possibly, why these drugs have a sedative action without affecting the consciousness. Their mode of action depends on their relation with the metabolism of the monoamines. The alkaloids of Rauwolfia, and the derivatives of the phenothiazines, modify the tissue exchanges of 5-hydroxytryptamine (serotonin), noradrenaline and 3-hydroxytyramine (dopamine), but we know nothing whatsoever about the mechanism of this change. Nevertheless, it may be stated that there is a basic relationship between sedation and the alteration in the monoamine content of the brain.
The neuroleptics are characterized, therefore, by
a psychosomatic sedation, without affecting the consciousness;
an antipsychotic sedation producing a favourable effect on the symptomology of psychoses;
an effect on the neurovegetative system: they produce stimulation of the parasympathic probably by depression of the sympathetic;
an effect on the extrapyramidal nervous system.
Consequently they may all be used:
to produce a psycho-relaxing effect in states of fear, agitation or psychic excitation, when administered in small doses;
to produce an antipsychotic effect in the case of serious, confusional or aggressive psychic disorders, illusions and hallucinations, when administered in large doses.
Tranquillizers
Because of their numerous applications, tranquillizers are extremely useful in medicine. They are characterized by a sedative and anxyolitic action, which is free from hypnogenetic effects. Their mode of action somewhat resembles that of the barbiturates but they differ from the latter by the wide gap which separates the sedative dose from the hypnotic dose. Furthermore, when used as sedatives they have no effect whatsoever on the consciousness, respiration or circulation. It is not yet known what biochemical changes they produce, but their pharmacological behaviour seems to be due rather to electric and neurophysiological phenomena. They appear to act on the limbic system, which determines the individual's affective behaviour, including aggressivity, agitation, emotivity and sexuality.
The dose required to produce an effect on the limbic system is so small that it has no effect on the other parts of the brain, such as the reticular substance. This probably explains why it has no effect on the state of consciousness.
On the other hand, the exact point at which the tranquillizers attack remains so far unexplained. It is reasonable to suppose that by some other means they also inhibit inter-neuron communications in the central nervous system. This assumption would provide a valid explanation of their relaxing effect on the musculature.
As for the medical uses of these drugs, they are legion. They are justified wherever a sedating, relaxing and anxyolitic effect is required.
While on the subject of tranquillizers, a few words concerning alcoholism and its treatment would not be irrelevant.
A comparison between alcoholism and drug addiction reveals several common characteristics, the basis of which is an imperative need for self-modification.
Alcoholism corresponds to the general definition of addiction formulated by WHO. Abstinence syndromes present similar withdrawal phenomena to those produced by the hypnotics.
In addition to specific treatment with Antabuse, tranquillizers are effective in counteracting motor agitation during pre-delirium syndromes or delirium tremens. In view of their low toxicity, and the fact that they do not potentiate the effects of alcohol, chlordiazepoxide and meprobamate are extremely effective in cases of delirium tremens. Where agitation is severe, they are administered intraveneously, while the intramuscular method is chosen whenever rapid sedation is not required.
Furthermore, tranquillizers greatly facilitate control of withdrawal phenomena, both during and after withdrawal. By removing psychic tension they also help the patient to return to a more normal existence and abstain from further intake.
Stimulants of mood
As their name implies, these drugs stimulate the mood and correct depressive conditions. They consist of two important groups: MAO inhibitors and tricyclic derivatives which, since their mode of action is not identical, we shall consider separately:
MAO inhibitors
These drugs have both a stimulating and inhibiting effect and are used in psychiatry to cure depressions.
Their action is characterized by the blocking, in vivo, of the monoamine oxidase (MAO). This ferment, which is found in several organs, also exists in the brain, where it catalyses the deamination of various biogenic amines such as catecholamine, tyramine and tryptamine. By blocking the MAO, these drugs produce the following changes in the metabolism of the monoamines in the brain:
increase in the 5-hydroxytryptamine (serotonin), noradrenaline and 3-hydroxytyramine (dopamine);
reduction in the substances produced by the metabolism of these monoamines;
antagonistic effect of reserpine and its homologues.
Since MAO inhibitors are long-acting, they may present certain dangers due to accumulation. Their antidepressent action is undoubtedly due to blocking of the monoamine oxidase as the following tests demonstrate:
all the derivatives of hydrazine, characterized by the blocking of this ferment, have an antidepressent action;
all the derivatives of hydrazine, characterized by a very small or non-existent blocking effect on the MAO, have only an insignificant or nonexistent antidepressent action;
the derivatives which, though belonging to a different group from hydrazine, have a blocking effect on the MAO, are also effective as antidepressents;
antidepressent action is strictly connected with the blocking of the MAO and not with the blocking of related ferments. The inhibitors of other ferments (diaminooxydases, decarboxylases, DPNases, etc.), not related to MAO, have no antidepressent effect.
Tricyclic derivatives
This group of drugs, which is chemically related to phenothiazines, is linked pharmacologically either with the latter or the MAO inhibitors.
One of the main characteristics of the thymoleptics is to strengthen the action produced by the injection of biogenic amines.
However, the mechanism of their action on the central nervous system remains obscure.
Their medical use is the same as that of the MAO inhibitors.
Amphetamines or stimulants of alertness
Chemically related to adrenaline and ephedrine, the amphetamines are characterized by their direct action on the central nervous system, more precisely the cerebral cortex, in which they produce stimulation, accompanied by a weaker sympathomimetic peripheric effect. It may be demonstrated that preparations with a pharmacological behaviour simular to that of ephedrine strengthen the adrenaline effect provided, however, that this surrenal principle is present. If not, even with a strong dose, the action is nil.
Owing to their effect on the central nervous system, the amphetamines remove the feeling of fatigue, and produce an euphoric effect. Moreover, they have a depressent effect on the nerve centres which govern the appetite.
Apart from their action on the medulla oblongata, these substances antagonize the action of numerous depressent drugs, counteracting their paralysing effect on the central nervous system. They are successfully used in cases of circulatory deficiencies of a central origin. However, if the cause is peripheric, they are less effective.
Intoxications caused by narcotic substances such as the barbiturates are often characterized by a respiratory paralysis or deficiency of a central origin. Relatively large doses of analeptics are then necessary to combat this kind of poisoning. In addition to these antidote effects, amphetamines are also used for certain purposes in the therapy of obesity, certain hypertensions and spasms of the pylorus or the intestines. However, compared with the other substances dealt with in this survey, their medical use remains limited.
Hallucinogens
As we have seen, the hallucinogens are characterized by their common action, which varies only in intensity. We shall refer here only to lysergic acid diethylamide (LSD) which is by far the most active and has the same characteristics as all the others.
LSD has never been marketed in Switzerland. Its distribution must therefore be limited, the drug being used only for psychiatric investigation.
The pharmacology of LSD is connected with that of 5-hydroxytryptamine or serotonin. Derived from the nutritive amino acid known as tryptophane, serotonin is synthesized by the body in the proportion of 1 per cent, directly at the level of the different organs where it is found, such as the brain, liver, intestines, etc. It is found there in a compound form and released only when required, then immediately inactivated by the monoamine oxidase, a specific ferment responsible for this enzymatic decomposition. The substances which inhibit the action of this enzyme therefore reduce the rate of decomposition of the serotonin and indirectly increase the amount of this substance in the tissues.
Cervical serotonin is found in particular in the upper parts of the brain stem, the mesencephalon and the diencephalon, where its role is still obscure. Nevertheless, it may be stated that LSD is a powerful antagonist of 5-hydroxyrtyptamine. An attempt has been made to explain the hallucinations it causes by its antagonism to seratonin at the level of the central nervous system. But this explanation is still inadequate to understand the effect of the drug on the central nervous system. Moreover, the possibility of LSD having other points of impact on the functions of serotonin is not excluded.
The pharmacology of LSD has been studied by tests carried out on animals. Three groups of effects may be distinguished: peripheric, central and parasympathetic. These are described below:
Peripheric effects:
increase in uterine contractility identical to that caused by ergonovine;
contraction of the unstriated muscles of the vascular tissues, following large doses of LSD;
marked antagonism to serotonin; on the other hand, absence of any adrenolytic action of the ergotic alkakoids.
Central effects:
LSD is responsible for a host of reactions, some sympathetic, others parasympathetic, as follows:
mydriatic action, which may be inhibited by adrenosympathicolytic agents;
hyperglycemia in the rabbit, inhibited by the hydrogenated ergotic alkaloids;
strong action on the thermoregulatory centres, reflected by an increase in body temperature. This action may be inhibited by acting under narcosis with prior treatment by the hydrogenated ergotic alkaloids of the ergotoxine group;
increased piloerection resulting from the increased activity of the sympathetic system.
Effects on the vagus or parasympathetic:
increase in salivation and lacrimal secretion;
bradycardia;
inhibition or stimulation of respiration, in doses of 10-50 gammas/kg. Larger doses produce a stoppage of the heart.
Used in human medicine in combination with psychotherapy, LSD leads to an improvement in the condition of certain patients suffering from functional disorders of a non-psychotic nature. However, its use in therapy can cause serious complications:
in hysterical or paranoidal persons, it causes prolonged psychotic reactions;
it may cause disturbances in social behaviour, with loss of all sense of guilt;
it may produce dependence on account of its euphoric properties, and thus increase the tendency to suicide.
Consequently, the therapeutic use of LSD remains extremely limited. On the other hand, it facilitates psychiatric research, e.g., in the diagnosis of schizophrenia. Von Felsinger and his colleagues 4 believe that LSD is capable of producing an exacerbation of the symptomatology in cases of schizophrenia. More recently, in 1962, Anastasopoulos and Phothiades 5 demonstrated that schizophrenic patients have a more marked tendency to produce pathological responses under its influence.
LSD has been recommended in cases of neuroses which do not respond to psychoanalysis. During the period of intoxication, the patient will remember events going back very far in time, sometimes even to his birth. The results, discussed a few days later, may help to solve certain cases which have resisted psychoanalysis. Nevertheless, LSD is used in therapy only when all other methods of treatment have failed.
Curiously enough, LSD intoxication produces a lesser symptomatic effect among sick persons than among healthy persons. This fact reduces its therapeutic usefulness to some degree, especially as the results are scarcely any better with substantially large doses (100 and 130 gammas.) The question arises whether the psychoses mask the effect of the drug or whether they increase tolerance of it. The results obtained are therefore not always conclusive and they are often very difficult to interpret: it is, in fact, impossible to affirm whether the symptoms observed are due to the LSD or simply to the patient's schizophrenia. On the other hand, such treatment has never been known to aggravate the illness.
It is useful to note that LSD acts in extremely small doses, the threshhold of action being between 10 and 20 gammas. A dose of 30 gammas is already considered active. Compared with other drugs, it is:
1/100 that of pervitine;
1/500 that of mescaline;
1/5,000 that of cocaine;
1/500,000 that of ethylalcohol.
In concluding this chapter, we may note that as far as these substances are concerned, medical practice in Switzerland does not differ greatly from that in most other parts of the world.
However, as regards clinical experiments with the new substances, certain differences relating rather to form than substance may be noted. Although the regulations governing the strictness of these experiments are quite comparable, there are certain differences in the manner in which the experiments are carried out.
Von Felsinger, J., Lasagna, L., Beecher, H., "The responses of normal men to lysergic acid derivatives ", J. Clin., Exp. psychopath. 17, 414-28 (1956).
Anastasopoulos, G., Phothiades, H., "Effects of LSD-25 on relatives of schizophrenic patients ", J. Ment. Sci., 108, 95-98 (1962).
We shall revert to this question in the next chapter, which deals more specifically with control, when the Swiss regulations in this fields will be compared with those of other countries such as France, the United Kingdom, the United States and the USSR.
As for medical science, the object, both here and abroad, remains the same, namely, to discover increasingly more active and less harmful derivatives. Thanks to the collaboration of physiology and biology, on the one hand, and pharmacology and clinical practice on the other, research is less and less empirical and is becoming more precise, more direct - more costly, perhaps - but undoubtedly more effective.
THE HISTORICAL BACKGROUND
Before we consider the problem of the control of psychotropic drugs as it arises in Switzerland, we feel that it is necessary rapidly to review our health legislation - which reflects the federal character of the country.
The federal Constitution does not confer on the Confederation any right to legislate in the field of drugs. This is the direct prerogative of the cantons. Nevertheless, provision has been made for certain exceptions, because the Confederation has been declared competent in certain specific sectors, which include, in particular, serums and vaccines, alcohol and narcotic drugs. However, the Confederation's authority in the matter of narcotic drugs has been transferred to the cantons which, under federal supervision, exercise control inside the country, while customs, imports and expert matters remain the concern of the Confederation.
Although numerous attempts to achieve uniformity were made in the last century, they all ended in failure. However, by 1900 the need for uniformity in the field of drugs was already beginning to be felt more acutely with the rapid development of the chemical industry and the appearance of the first pharmaceutical preparations. Thus was established, with the approval of the Federal Council, the Inter-cantonal Drug Control Convention, whereby the Swiss cantons formed a public corporation having legal status and headquarters situated at Berne, whose aim is to simplify and facilitate drug control, through the Inter-cantonal Drug Control Office, more commonly known as OICM.
By acceding to this Convention, the Swiss cantons undertook, on the one hand, not to market drugs which did not meet the specifications of OICM, and on the other, to adapt their health legislation to the latter's requirements. However, OICM has no legislative or coercive powers over the cantons; its role is to make recommendations which the latter may either adopt or reject, as they wish.
Owing to the nature of our federalist system, the adaptation of the cantonal legisation has proceeded in stages. Today, however, this process may be said to be virtually complete, the cantons in whose territory pharmaceutical undertakings have their headquarters having adopted the OICM standards.
Before describing the responsibilities of OICM, we should like first of all to consider rapidly its structure and its main component organs. These are:
The assembly of cantonal delegates. This is the supreme organ in which each canton is represented.
The governing board, which exercises supreme control over the Office, is primarily concerned with preparing the business, dealt with by the assembly, the appointment of the director, members of the college of experts and the staff of the Office.
The college of experts is composed of university professors specialized in the various scientific disciplines. They perform their OICM role outside their normal duties and assume the highest degree of responsibility for the decisions taken.
The director.
The appeals committee.
The auditors.
The Office's tasks are manifold. They are defined in the Inter-cantonal Drug Control Convention of 16 June 1954 and in the OICM Regulations of 10 June 1955 and they involve carrying out expert appraisals of the various drugs, medical appliances and supplies and then registering them in accordance with the criteria indicated below.
In assessing a drug or pharmaceutical preparation, the experts' main concern is the patient's health; consequently, they ensure that the latter, or simply the purchaser, receives precisely what the manufacturer claims he is supplying. All expert appraisals are based, therefore, on an analysis of the drug concerned and must extend to its chemical, micro-chemical physicochemical and pharmacognosic aspects as well as its biological or micro-biological characteristics.
Editorial Note:
In Switzerland, as in many other countries, which manufacture pharmaceutical products, including narcotic drugs and suchlike psychotropic substances, measures have to be taken to ensure that the products manufactured are safe and effective. The description of the role and organization of the Swiss Inter-Cantonal Drug Control Office is useful in appreciating the various elements that must be taken into account in this work.
The first group of analyses is carried out directly in the OICM laboratory, so far as the composition and conservation of the drug are concerned. Attention is also paid to determining the degree of purity of the substance and to verifying that its composition corresponds exactly with the stated formula.
It is also important to verify that the drug will not deteriorate with time, since a preparation may quite well possess all the required properties at the time of manufacture, but gradually lose them through faulty conservation. It may even show a certain amount of toxicity due to the transformation of internal reaction of some of its components.
This leads to an examination of factors which relate neither to the composition, nor to the pharmaceutical form of the substances themselves, but solely to their containers, the material of which must be entirely safe for the drugs they hold.
OICM entrusts the biological or micro-biological analyses, which call for special laboratory techniques, to Swiss university institutes that are particularly well equipped for such research. Thus vitamin products are analysed by the Vitamin Institute at Basle, while those of hormonal composition are entrusted to the Swiss Hormone Institute at Lausanne.
The results of these expert appraisals, together with any necessary recommendations, are then transmitted by these institutes to OICM, for final decision and action.
As already indicated, the expert appraisal of each drug is the prime objective of any examination carried out by OICM. The appraisal must therefore be very comprehensive and cover all the following aspects:
composition;
usefulness;
efficacity;
toxicity;
side effects;
method of sale;
publicity;
selling price.
Naturally, such expert appraisals take a relatively long time, since an assembly line-type of operation, such as is normally carried out in industry by the use of appropriate methods, is impossible in such a central control laboratory. The variety of products submitted for analysis is so great that the problems involved and the research they entail are inevitably different in each case.
We might now consider the OICM requirements which must be met by all manufacturers submitting new substances for expert appraisal. These requirements are of relatively recent date, having first been established early in 1963. As a result of the accidents caused in 1962 by the drug thalidomide, drug control measures were tightened throughout the world. It may be interesting to consider, therefore, the measures taken in Switzerland, and to compare them briefly with those of the other main drug-producing countries.
OICM's requirements are of two kinds; those relating to preliminary tests, and those relating to clinical examinations.
Preliminary tests
Before being applied to humans, all new substances must be tested on animals. These tests should generally extend over a period of more than two years. The ratio of substances tested to those subsequently used is in the region of 3000:1, so that each year, only 50 new drugs are placed in the service of medicine in the western world, while more than 150,000 are tested.
The tests relate both to the chemical stability of the new derivative and its pharmacological action, absence of or relative toxicity, reabsorption by the organism, blood concentration, mode of elimination, possible teratogenesis and side effects.
Clinical examinations
These examinations constitute the second stage in the testing of any new substance. The tests are carried out first of all on a very limited scale, on volunteers, in order to determine the thresholds of action and toxicity for humans. It is only after the substance has successfully passed these tests that more extensive clinical examinations can be considered. These are organized along the following general lines.
Verification of the efficacy of the new drug or its maximum efficacy compared with that of others used in the treatment of the same complaint.
Delimitation of its efficacity. Is it more effective than similar drugs?
Determination of its range of application.
Determination of contra-indications.
Determination of the minimum and maximum doses, the initial dose, and the regular dose. Establishing the posology.
Fixing the duration of therapy.
Investigation of side effects:
in therapeutic doses,
in the event of an accidental overdose.
Investigation of the synergic or antagonistic effects vis-a-vis other substances.
For these investigations, use is made of the new substance alone, or of the alternate application of placebos or the method comprising two unknowns.
These clinical data are based on the study of several hundred cases, sometimes as many as 2,000. Clearly, such research takes a relatively long time - not infrequently, in the case of new drugs such as the oral antibiotics or certain antiepileptics, over two years. Generally speaking, this undoubtedly causes some delay in production. However, in view of the rapid growth of drug production, particularly in the last decade, the requirements imposed by the State appear to be fully justified for the State is after all the custodian of public health. Such is the price of safety...
The measures adopted in Switzerland differ only very slightly from those employed in other countries. Compared with the methods used in the United States of America and, in the case of Europe, in France, the United Kingdom and the USSR, the basic tests are identical. The only differences relate to questions of application.
For instance, in the United States, the Food and Drug Administration (FDA), a national agency responsible for drug control, requires that medical practitioners and hospitals carrying out clinical examinations shall hold a special licence.
The same principle applies in the USSR, where control is exercised by a pharmacological committee appointed by the Ministry of Health.
In France, the study of the records pertaining to any new drug is carried out by a committee of experts composed of specialists in the various scientific disciplines.
Its United Kingdom counterpart is the Dunlop Committee, which has three sub-committees - the first responsible for toxicological tests, the second for clinical tests and the study of therapeutic efficacy, and the third concerned with possible contra-indications. In addition, precise statistics are required of the results obtained with new products, as compared with those yielded by placebos or other substances, with which the drugs are compared.
Accordingly, as already stated, the control measures called for in Switzerland by OICM are practically the same as those required in every other country; the only difference being the greater flexibility with which the clinical tests are carried out. This, we feel, cannot detract in any way from their value.
After the drug has successfully passed all the required tests, it becomes the subject of an experts' report containing all relevant information, and confirming, for the benefit of the cantonal authorities, that the drug has been registered with OICM. It also specifies the method of sale, by classifying the substance in one of the following groups, identified by appropriate labelling:
Group A: Drugs sold exclusively in pharmacies, on medical prescription, without refill, ne repetatur (NR).
Group B: Drugs sold exclusively in pharmacies on medical prescription, with refill.
Group C: Drugs sold exclusively in pharmacies, without a medical prescription.
Group D: Drugs sold in pharmacies and dry-salters shops (drogueries)
Group E: Drugs sold in all shops.
The placing of a medicinal substance in one of the above five categories depends essentially on the following two factors:
the type of substance and its physiological action;
the preparation's field of application, which is essentially a public health factor.
Accordingly, a drug which is intended for use in treating a severe illness, such as tuberculosis, can be sold only on medical prescription, even if its chemical composition were to warrant a different method of sale.
Publicity is also subject to certain regulations. For the drugs in groups A, B, and C, this is strictly limited to professional advertising; only the drugs in groups D or E may be publicly advertised. Nevertheless, prior authorization by OICM is necessary in this case, in order to preclude any charlatanism or inaccuracy likely to mislead the public.
Drug registrations with OICM are valid for a maximum of five years. However, if in that time progress in science reveals a danger in the use of a particular component, the registration would, of course, be immediately reviewed and adapted to the new conditions.
The wholesale and retail drug trade is itself regulated at the cantonal level. No one may avail himself of the right to manufacture or trade in drugs, either as a wholesaler or retailer, without previously having obtained the authorization of the canton concerned. This is only granted when the necessary requirements, both as regards the premises used and the qualifications and competence of the personnel are met.
Furthermore, before any application can be made for registration of a drug with OICM, the authorization of the canton concerned must be obtained. Without this authorization no application can be entertained.
Having taken a general look at the drug control machinery applied in Switzerland, we now propose to examine the special regulations governing the group of substances with which we are concerned here. For control purposes, these may be divided into two groups: the hallucinogens on the one hand, and the amphetamines, barbiturates and tranquillizers on the other.
The question of the hallucinogens, mainly involving LSD and psilocybin, was already regulated at the national level last autumn, when these substances were assimilated to narcotic drugs. Since they are now subject to the stringent narcotics legislation, their abuse is virtually prohibited. Control in the case of the second group of drugs is based on their classification in one of the five OICM categories. The use of amphetamines, all of which are included in list A, is strictly limited to a single medical prescription, without refill. Barbiturates and tranquillizers (list B) are dispensed on medical prescription only, but refills are nevertheless permitted. However, some of these substances may be included in list C if the percentage of active principle is low and there is consequently no confirmed public hazard (if the drug were later found to constitute a potential health hazard, it would be placed in list B). Accordingly, the main criterion adopted by OICM is not so much a drug's composition or pharmacological action as its danger to public health. This, however, can only be recognized after the product has been marketed, and in the light of experience acquired in its handling.
Clearly, the effectiveness of such a system will depend, above all, upon strict observance of restrictions by those responsible for the sale of the drugs: any slackness in applying the OICM directives may lead to certain abuses. However, as already indicated, it is very difficult to say whether such a situation actually exists without knowing the results of the extensive investigation now being conducted by the Kielholz Commission, at the request of the cantonal directors of public health. These results will enable us to obtain a more objective view of the problem.
Cf. "Narcotics Control in Switzerland" by Bertschinger J. P. elal."Bulletin on Narcotics, Vol. XVI, No. 2, April-June 1964 pp. 1-16.
Whatever the results may be, and with no knowledge as yet either of the extent or of the urgency of the problem, there would seem to be little point in giving a detailed definition of the actual notion of abuse. The latter may be due to three different causes:
It may result from the immoderate and repeated use of drugs unlawfully obtained, by whatever means;
It may, on the other hand, be due to drugs obtained quite lawfully and be of entirely licit origin, as a result of subsequent exceeding of the doctors' orders;
It may be the result of a medical prescription too readily given.
Now that the question of hallucinogens is regulated in Switzerland and consequently separated from the rest of the problem, we feel that the present system of control should meet our expectations with the efficacy and flexibility desired.
In our view, it would be extremely difficult to equate all psychotropic substances with narcotic drugs without further qualification. First of all, it would be necessary to distinguish between dependence-producing drugs and non-dependence-producing drugs. Except for the group of amphetamines, which are limited in number and have relatively few therapeutic uses, control of the type applied to narcotic drugs would be very difficult to achieve for all psychotropic drugs liable to produce addiction. In view of the numerous uses and very large number of these drugs, control would necessitate an extremely cumbersome administrative machinery, disproportionate to the importance of the problem itself, and virtually impracticable at the level of the retailer.
For this reason, we feel that the adoption of the OICM classification for the second group of substances represents a practical and sufficiently effective system. If certain drugs dispensed on prescription with refill are shown to be the cause of abuse, they can quite easily be transferred to list A where, like the amphetamines, they will be obtainable on medical prescription only, without refill. Furthermore, if observance of these restrictions proved to be inadequate, more severity could be shown towards offenders.
The whole problem deserves broader treatment, based on the systematic education of the public. Obviously, abuse is determined by the public's own demand for drugs. In view of the importance now attached to the popularization of medical knowledge, it is not enough to make the public mildly curious about a particular drug, or to provide it with a completely superficial and relative knowledge about it. Its attention must also be drawn to the many risks and certain dangers it incurs by the abuse or misuse of the drug. Requests to physicians would then certainly become less pressing and the latter would be spared having to write out prescriptions which they sometimes give only to oblige the patient. To be complete, such education should be accompanied by the education of the physician himself. Although in general the medical profession is perfectly aware of the dangers connected with the prescription of a particular group of drugs, it is equally obvious that a certain minority of physicians seems quite oblivious of the very existence of any problem. What use would information at all the other levels be if the very source of the prescription sometimes failed to recognize the danger or simply assessed it incorrectly? Therefore the solution of the problem cannot be found in control alone. However perfect the latter may be, it can never be anything but a single though important side of a triptych, the other panels of which are education of the public and realization by the entire medical profession, as well as by the pharmaceutical profession and industry, of their own particular responsibility.
As regards the foreign trade in this field, Switzerland is primarily an exporting country and imports only a relatively small percentage of the drugs used in its territory. All imported drugs are compulsorily registered with OICM, except in the case of a drug imported exceptionally for an individual consumer on the direct responsibility of a physician or pharmacist.
As regards exports, the only drugs which may be exported are those duly registered in Switzerland (even if, for all practical purposes, these were intended for export only). Of course, responsibility regarding import formalities and the use of the drugs rests entirely with the importing country: the latter may in every case request certification of the actual use of the drug in the manufacturing country, i.e., Switzerland, and, in practice a large number of importing countries take this precaution.
Clearly, however, neither OICM nor any other Swiss body can influence the use which is made of drugs once they are exported and it is thus entirely the responsibility of the importing country, having been duly informed, to apply any measures it deems necessary (one possible suggestion would be to adopt the practice followed in the exporting country as regards distribution of the drugs).
On examining the problems arising in connexion with the drugs dealt with in this article, we see that they are not uniform or even of equal intensity throughout Switzerland. Owing to the configuration of the country and the fact that a higher density of population is found in the urban regions of the Plateau, consumption is by no means comparable in all cantons. Although, as indicated earlier, there are as yet no accurate data concerning the use of these drugs, the major consumption obviously takes place in the large towns - as opposed to the more sparsely populated rural or mountainous regions - where the pace of life is naturally more conducive to their use. On the other hand, consumption in the industrial towns does not differ much from consumption in the tourist centres, since the effect of the industrial element in the former is usually counterbalanced by the tourist element in the latter. As a result, consumption in a limited number of cantons reflects approximately the consumption of the entire country. In an interesting study published in 1960, J. P. Bertschinger established the total value of drugs consumed in Switzerland in the years 1953-1957 at 360 millions francs, i.e., 70 francs per inhabitant per annum. Bearing in mind the increase in population, the figure today might stand somewhere in the region of 400 million francs, i.e., roughly 80 francs per inhabitant per annum. It would be most interesting to know, once the report of the Kielholz Commission has appeared, what is the share of psychotropic drugs in the total consumption.
In conclusion, what implications would the international control of such substances have for Switzerland? We may leave aside the hallucinogens and LSD, because these are covered in Switzerland by the narcotics legislation.
As far as amphetamines are concerned, stringent control measures in respect of those most commonly used would not, a priori, present any major difficulties since, after all, their application in the medical field remains limited and the number of derivatives is relatively small. This would not apply, however, to the barbiturates and still less to tranquillizers, in the wider meaning of the term; for these have countless derivatives, are used extensively for therapeutic purposes and only some of them are liable to produce dependence. The use of barbiturates would even appear to be declining in Switzerland, their place being taken by tranquillizers. However, strict control of these substances, with registration of imports and exports would in our opinion, be difficult to achieve. The administrative machinery needed would create serious difficulties at all levels, especially at the pharmacy level. The present system, under which the presentation of a medical prescription is compulsory, and even the number of dispensations must be limited for certain types of prescription, seems infinitely preferable and more practical. We consider the existing safeguards adequate, both for limiting possible abuse and for preserving public health.
H. A. Abramson, "Lysergic acid diethylamide (LSD-25): The questionnaire technic with notes on its use ", The Journal of Psychology, 49, 57-65 (1960).
J. de Ajuriaguerra, P. Dick, R. Tissot, "Les médicaments psychotropes et leur action thérapeutique", Service scientifique Roche (1966).
W. Alhadeff, "Aspects chimiques de l'emploi du dlisyde et de lindocybine en psychiatrie," Journal suisse de pharmacie, 245-9 (1963).
E. F. W. Baker, "The use of lysergic acid diethylamide (LSD) in psychotherapy, The Canadian Medical Association Journal, 91, 1200-2 (1964).
R. Battegay, "Comparative investigations of the genesis of alcoholism and drug addiction ", Bulletin on Narcotics, XIII, 2, 7-17 (1961).
J. P. Bertschinger and V. Arni, "Arzneimittelumsatze im Ausland und in der Schweiz", Schw. Apoth. Zeitung, 11, 179-182 (1960).
J. Büchi, "Die Pharmaka des autonomen Nervensystems ", Subs. Pharm., 1-42 (1957).
M. de Buman, "Essai du librium en obstétrique ", Méd. et Hyg., 19, 835 (1961).
A. Cerletti, "Pharmakodynamische Grundlagen zur differenzierten Psychopharmakotherapie ", Gerontolgia Clinica Addit., 123-137 (1962).
A. Cerletti, Biochimie, physiologic et pharmacologic de la 5-hydroxytryptamine - Méd. et Hyg., 16, pp. 228-231 (1958).
A. Cerletti, "Quelques aspects nouveaux de la pharmacologie du système nerveux végétatif", Méd. et Hyg., 15, 6 (1957).
A. Cerletti, "Die Bedeutung der Indole im Zentral-Nervensystem - Die Umschau in Wissenschaft und Technik ", Sonderdruck, Heft 2 (1963).
A. Cerletti, E. Schlager, F. Spitzer, M. Taeschler, "Psycho-pharmaka ", Subs. Pharm., 313-314 (1963).
S. Cohen and K. S. Ditman, "Prolonged adverse reactions to lysergic acid diethylamide ", Arch. of General Psychiatry, 8, 475-80 (1963).
S. Cohen and K. S. Ditman "Complications associated with lysergic acid diethylamide (LSD-25)", The Journal of the American Med. Assoc., 181, 161-2 (1962).
Economic and Social Council, Substances not under international control (United Nations). Doc. E.C.N. 7/AC.6/3
J. Druey, "La recherche dans l'industrie pharmaceutique suisse", Pharma Information (1966)
J. Fort, "The problem of barbiturates in the United States of America ", Bulletin on Narcotics, XVI, 1, 17-35 (1964).
M. Glatt, "The abuse of barbiturates in the United Kingdom' Bulletin on Narcotics, XIV, 2, 19-38 (1962).
C. Gruhzit, "Pharmacological investigation and evaluation of the effects of combined barbiturate and heroin inhalation by addicts ", Bulletin on Narcotics, X, 3, 8-11 (1958).
C. Gruhzit and C. O. Lee, "The use of meprobamate in the treatment of heroin withdrawl symptoms", Bulletin on Narcotics, XI, 2, 6-14 (1959).
F. Hurlimann and W. Pulver, "Die Basis Therapie des Delirium tremens mit Chlordiazepoxid ", Schweiz. med. Wschr., 95, 46, 1591-6 (1965).
A. Hofmann, "Chemical, pharmacological and medical aspects of psychomimetics" J. Expt. Med. Sc., V, 2 (1961).
H. Isbell, "Abuse of barbiturates ", Bulletin on Narcotics, IX, 2, 14 (1957).
H. Isbell, "Comparison of the reactions induced by psilocybin and LSD-25 in Man, Psychopharmacol., 1, 29-38 (1959).
A. Jenni and A. Pletscher, " Monoamine oxydase-Hemmer ", Subs. pharm., 219-231 (1962).
R. Kappel, "überlegungen zur Geschäftspolitik der Export-unternehmen der schweizerischen chemischen Industrie", Pharma-lnformation (1964)
E. Koss, N. Retterstol, T. Sirner, "Barbiturate intoxication and addiction as a public health problem in Oslo ", Bulletin on Narcotics, XI, 3, 15-28 (1959).
N. S. Kline, "Thérapeutique de la dépression - Tables psycholeptiques pour praticiens", 5-10, Edit. Méd. et Hyg.
G. Lai and E. de Perrot, Le librium dans le traitement du delirium tremens ", Praxis, 51st year, 9, 236-8 (1962).
H. Leuner, "Die psycholytische Therapie: Klinische Psychotherapie mit Hilfe von LSD-25 und verwandten Substanzen ", Zeitschr. f. Psychotherap. und med. Psychol. - Sonderdruck Heft 2 (1963).
H. Leuner and H. Holfeld, "Ergebnisse und Probleme der Psychotherapie mit Hilfe von LSD-25 and verwandten Substanzen ", Psychiatr. neurol., 143, 379-391 (1962).
L. Levi, "The barbituric acids, their chemical structure, synthesis and nomenclature ", Bulletin on Narcotics, IX, 1, 30-40 (1957).
D. Loew, "Intoxications aigues dues aux médieaments psychotropes", Tables psychol. pour praticiens, 11-13, Edit. Méd et Hyg.
J. Montanez Salcedo, "Pharmacodynamie comparée des méthamonodiazepoxides ou librium et du méprobamate", Thesis No. 2789, Faculty of Medicine, Prof. E. Frommel, University of Geneva (1961).
K. Morimoto, "The problem of the abuse of amphetamines in Japan", Bulletin on Narcotics, IX, 3, 8-12 (1957).
K. Neuhold, M. Taeschler, A. Cerletti, "Beitrag zur zentralen Wirkung von LSD: Versuche über Lokalisation von LSD Effekten ", Helv. Physiol. Acta, 15, 1-7 (1957).
R. J. H. Oberholzer, "Die klinische Prüfung neur Arzncimittel ", Pharm. Act. Helv., 465-479 (1964).
B. Pelimont, F. A. Steiner, H. Besendorf, H. P. Bochtold and E. Lauppi, "Introduction à la pharmacologie d'un nouveau neuroleptique d'action particulière: le Taractan, Schweiz. med. Wochenschr., 90, 22, 598-599 (1960)
A. Pletscher, "Zentrale Wirkungsmechanismus von Psychopharmaka", Sympos. Wien 1963, 25-42, Karger, Basel/ N.Y. (1964).
Report of the Sub-Committee on Narcotics: "Illicit traffic in narcotics, barbiturates and amphetamines in the United States ", Bulletin on Narcotics, VIII, 3, 13-18 (1956).
E. Rothlin and A. Cerletti, "Pharmacology of LSD-25 ", Extr. from Lysergic acid diethylamide and mescaline in experimental psychiatry, Grune and Stratton, Inc. (1956).
R. A. Sandison and J. D. A. Whitelaw, "Further studies in the therapeutic value of lysergic acid diethylamide in mental illness ", The J. of Mental Science, 103, 431 (1957).
G. Seidman and J. C. Kenna, "The use of LSD-25 as a diagnostic acid in doubtful cases of schizophrenia ", The Brit. J. of Psych, III, 470 (1965).
W. A. Stoll, "Lysergsäure-diathylamid, ein Phantastikum aus der Mutterkorngruppe ", Schweiz. Archiv. f. Neurol. u. Psych., IX (1947).
M. Taeschler and E. Schlager, "Neuroleptica ", Subs. pharm., 168-192 (1962).
M. Taeschler and E. Schlager, "Tranquillizer ", Subs. pharm., 193-218 (1962).
M. Tchicaloff, "La thérapeutique de l'épilepsie ", Subs. pharm., 623-638 (1966).
R. Tissot, "L'action thérapeutique de quelques drogues psychotropes vues à travers leurs propriétés phaxmacologiques ", Serv. scient. Roche (1966).
J. Tripod, "Mesures prises actuellement en faveur de la sécurité des medicaments ", Pharma Information (1965).
W. Weiss and H.P. Jaspersen, "Die Antihistaminika", Subs. pharm., 61-81 (1960).