Background
Tryptamine and its derivatives that have been reported as NPS are indolealkylamine molecules. While some naturally occuring tryptamines are neurotransmitters (e.g. serotonin, melatonin and bufotenin), most are psychoactive hallucinogens found in plants, fungi and animals (e.g. N,N-dimethyltryptamine (DMT) psilocybin, and 5-methoxy-N,N-dimethyltryptamine (5-MeO-DMT) [1-3].
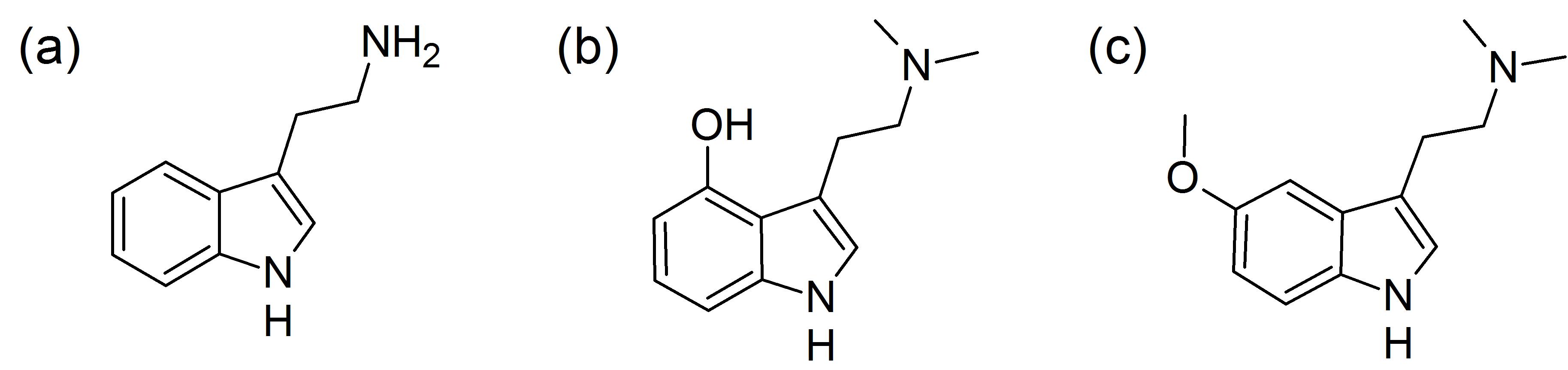
Figure 1 Tryptamine and related compounds: (a) tryptamine, (b) psilocin and (c) 5’-methoxy-N,N-dimethyltryptamine (5-MeO-DMT)
The use of the naturally occurring psilocybin, became widespread in the late 1950s in the United States, whereas synthetic tryptamines appeared on illicit drug markets only throughout the 1990s. Recently, a group of synthetic tryptamines that are derived from DMT and other naturally occurring tryptamines have been reported as NPS, including 5-MeO-DMT, 5-MeO-DPT, AMT, 4-AcO-DMT and 4-AcODiPT
DMT, etryptamine, N,N-diethyltryptamine (DET), Psilocin and psilocybin, are the only tryptamines under international control (listed in Schedule I of the 1971 Convention), while some others are controlled at the national level in several countries.
Description
Street names for some tryptamines include ‘Foxy-Methoxy’ (5-MeO-DIPT); ‘alpha-O’, ‘alpha’ and ‘O-DMS’ (5-MeO-AMT); ‘5-MEO’ (5-MeO-DMT). Natural tryptamines are available in preparations of dried or brewed mushrooms, while tryptamine derivatives are sold in capsule, tablet, powder or liquid form. Tryptamines are generally swallowed, sniffed, smoked or injected.
Tryptamines act predominantly as hallucinogens. Classic hallucinogens (psychedelics) mediate specific serotonin-receptor activities and produce hallucinations. Substances in these group mimic the effects of traditional drugs such as 2C-B, LSD and DMT but may also possess residual stimulant activity.
Reported adverse effects
Toxicological studies on tryptamines remain limited. Reported adverse effects related to the use of ‘foxy methoxy’ include restlessness, agitations, gastrointestinal distress, and muscle tension [4]. Rhabdomyolosis after ingestion of ‘Foxy’ has also been described in a case study [5]. Other fatalities associated with the use of ‘Foxy’ and other tryptamines have also been described in scientific literature [6].
References
[1] Collins, M., “Some new psychoactive substances: precursor chemicals and synthesis-driven end-products”, Drug Testing and Analysis 3 (2011): 404-16.
[2] Bufotenin (a tryptamine closely related to serotonin) was originally found by Wieland in the 1930s; Wieland, H., Konz, W. and Mittash, H., “Die Konstitution von Bufotenin und Bufotenidin. Über Kröten-Giftstoffe VII”, Justus Liebigs Annalen der Chemie 513.1 (1934): 1-25.
[3] The structures of psilocin and psilocybin were confirmed by Albert Hoffmann et al. in 1959; Hoffmann, A., Heim. R., Brack, A. and Kobel, H., Experientia 14 (1958): 107-9; Hoffmann, A., et.al., “Psilocybin und Psilocin, zwei psychotrope Wirkstoffe aus mexikanischen Rauschpilzen”, Helvetica Chimica Acta 42 (1959): 1557-72.
[4] Alatrash, G., Majhail, N.S. and Pile, J.C., “Rhabdomyolysis after ingestion of “Foxy,” a hallucinogenic tryptamine derivative”, Mayo Clinic Proceedings 81.4 (2006): 550-1.
[5] Alatrash, G., Majhail, N.S. and Pile, J.C., “Rhabdomyolysis after ingestion of “Foxy,” a hallucinogenic tryptamine derivative”, Mayo Clinic Proceedings 81.4 (2006): 550-1.
[6] Einosuke, T., et.al., “A fatal poisoning with 5-methoxy-N, N-diisopropyltryptamine, Foxy”, Forensic Science International 163 (2006): 152–4; Sklerov, J., et.al., “A fatal intoxication following the ingestion of 5-methoxy-N,N-dimethyltryptamine in an ayahuasca preparation”, Journal of Analytical Toxicology 29.8 (2005): 838-41.